Solution:
The Structural Formula of Primary, secondary and tertiary amine of each from $C_3H_9 N$ are:
Propan-1-amine: $C$$H_3$-$C$$H_2$-$C$$H_2$-$N$$H_2$→ One Degree Amine
N-methylethanamine: $CH_{3}-NH-CH_{2}-CH_{3}$ → Two Degree Amine
N,N dimethylethanamine: $C{H_3} - \mathop N\limits^{\mathop |\limits^{C{H_3}} } - C{H_3}$ → Three
Degree Amine
Use Common Method for Separation:
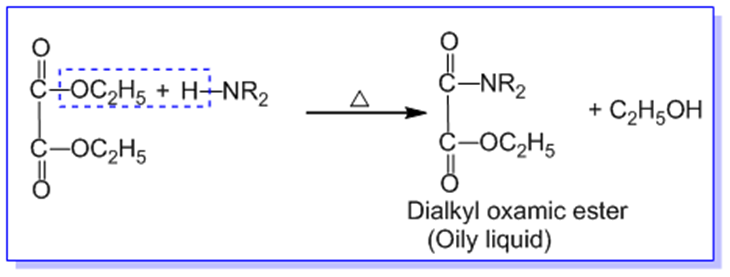
In Hoffmann’s method, the given mixture of primary, secondary, and tertiary amines is heated with diethyl oxalate when,
A. 10 amine forms dialkyl oxamide which is crystalline solid.
B. 20 amine forms dialkyl oxamic ester which is an oily liquid.
C. 30 amines do not react as it does not contain replaceable hydrogen atom on nitrogen.
The reaction mixture containing dialkyl oxamide, dialkyl oxamic ester, tertiary amine and ethyl alcohol is first filtered and solid product of dialkyl oxamide is separated. Dialkyl oxamide is heated with aq.KOH to recover primary amine.
The remaining mixture of dialkyl oxamic ester, tertiary amine and ethyl alcohol is subjected to fractional distillation. Tertiary amine is distilled out first. The residual dialkyl oxamic ester is heated with aq. KOH to recover secondary amine and alcohol in different fractions.
In this way, the given mixture of 10,20 and 30 amines are separated by Hoffmann’s method.