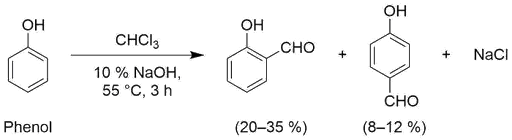
- What happen when phenol is treated with chloroform in presence of base.
- Prepare DDT , Benzene from chlorobenzene ,2 chloroacetophenone.
- Why phenol is more acidic than alcohol ? Give reason with structure.
- Phenol in presence of base (sodium hydroxide) reacts with chloroform to form salicyladehyde. The reaction is known as Reimer-Tiemann reaction. Reaction
-
DDT From Chlorobenzene:
DDT is prepared y heating chloral and cholorobenzene in 1:2 ratio in presence of Conc. H2SO4. Reaction:
 Benzene from Chloro Benzene:
Benzene from Chloro Benzene:
On reduction of chlorobenze with Nickel metal and Al/NaOH, benzene is formed. Reaction:
 2-chlaroacetophenone
Reaction:
2-chlaroacetophenone
Reaction:

- Phenol is more acidic than alcohol due to the presence of an electron withdrawing group (-OH) attached directly to the aromatic ring. This group stabilizes the conjugate base formed after losing a proton, making it easier to donate a proton.