2079 Chemistry Exam Paper Solution
Group ‘A’
Rewrite the correct option.
1. 2R-OH + 2Na → 2RONa +
H2 is example of:
i.
Acidic nature alcohol
ii.
Basic nature of alcohol
iii.
Electrophilic substitution reaction
iv.
Nucleophilic substitution reaction
Ans: Acidic Nature of Alcohol
2. C6H5CH2-CHO
and C6H5CHO can be distinguished by:
i.
Iodoform Test
ii.
Tollens’ Test
iii.
2,4 DNP test
iv.
Fehling Test
Ans: Fehling Test
3. An organic compound X
undergoes reduction with LiAlH4 to held Y. When vapor of Y are
passed over freshly reduced copper at 3000 Celsius X is formed. The
compound Y is
i.
CH3CHO
ii.
CH3CH2-OH
iii.
CH3-CO-CH3
iv.
CH3-O-CH3
Ans: CH3CH2-OH
4. The number of
possible isomers of 10 amines of a molecular formula C4H11N
give:
i.
1
ii.
2
iii.
3
iv.
4
Ans: 4
Explanation:
These arrangements can be
represented as:
a)
CH3-CH2-CH2-CH2-NH2
b)
CH3-CH2-CH(CH3)-NH2
c)
CH3-CH(CH3)-CH2-NH2
d)
CH(CH3)-CH2-CH2-NH2
5. Acetic anhydride is
obtained from acetyl chloride by the reaction of
i.
Conc.H2SO4
ii.
P2O5
iii.
CH3COONa
iv.
CH3COOH
Ans: CH3COONa
6. A metal (M) forms
thiosulphate with molecular formula M2S2O3.
The valency of metal is
i.
1
ii.
2
iii.
3
iv.
4
Ans: 1
7. A PH of 10-8
M aqueous solution of HCl is:
i.
Less than 7
ii.
7
iii.
8
iv.
More than 8
Ans: Less than 7
8. A catalyst
accelerates the reaction because
i.
It brings reactant closer.
ii.
It lowers the activation energy.
iii.
It increases the activation energy.
iv.
It forms complex with reaction.
Ans: It lowers the activation
9. What is the
concentration of nitrate ions if the equal volume of 1M NaNO3 and 1M
KCL are mixed
i.
0.25M
ii.
0.5M
iii.
1M
iv.
2M
Ans: 0.5M
10. Tailing pf mercury
is due to formation of
i.
HgO
ii.
Hg2O
iii.
HgO2
iv.
Hg2O2
Ans: Hg2O
11. Bell metal is an
alloy of
i.
Cu, Pb and Sn
ii.
Sn and Cu
iii.
Zn and Pb
iv.
Zn, Cu and Sn
Ans: Sn and Cu
Group B
12. An electrochemical
cell is constructed by using aluminum and silver electrodes whose electrodes
potential values are:
E0
Al3+/Al = -1.67V
E0
Ag+/Ag = 0.8V
a.
What is meant by electrochemical cell?
Ans: An electrochemical
cell is a device that converts chemical energy into electrical energy through a
redox reaction. It consists of two half-cells, each with an electrode immersed
in an electrolyte solution. The half-cells are connected by a salt bridge or porous
membrane, which allows the flow of ions to maintain electrical neutrality.
b.
Represent an electrochemical cell using
above electrodes.
Ans: The electrochemical
cell using aluminum and silver electrodes can be represented as:
Al(s) | Al3+(aq) || Ag+(aq)
| Ag(s)
c.
Write down complete cell
Ans: The complete cell
reactions can be written as
At the anode (oxidation): Al(s) →
Al3+(aq) + 3e-
At the cathode (reduction): Ag+(aq)
+ e- → Ag(s)
Overall reaction: Al(s) + 3Ag+(aq)
→ Al3+(aq) + 3Ag(s)
d.
Calculate the emf of
Ans: The overall cell
reaction for this setup can be represented as follows:
Al(s) + Ag+(aq) → Al3+(aq) +
Ag(s)
The emf (electromotive force) of
an electrochemical cell is given by the difference between the reduction
potential of the cathode and the oxidation potential of the anode.
E°cell = E°cathode - E°anode
E°cell = 0.8 V - (-1.67 V)
E°cell = 2.47 V
OR
Hess’s Law is
applied to calculate the different types of enthalpy
i.
Define Hess’s law of constant heat summation.
Ans: Hess's law of
constant heat summation states that the enthalpy change of a chemical reaction
is independent of the route taken from reactants to products.
ii.
What is meant by enthalpy of rection?
Ans: Enthalpy of reaction
(also known as heat of reaction) is the amount of heat energy absorbed or
released during a chemical reaction at constant pressure. It is denoted by ΔH
and has units of joules per mole (J/mol) or kilojoules per mole (kJ/mol).
iii.
Standard enthalpy of formation of H2O2(l)
and are H2O (l) are -188KJ/mol and -286KJ/mol respectively. What
will be the enthalpy change of the following reaction:
2H2O2(l)
→ 2H2O (l) + O2 (g)
Ans: The enthalpy
changes of a reaction can be calculated by subtracting the sum of the
enthalpies of the reactants from the sum of the enthalpies of the products,
with the coefficients of each species in the balanced equation considered. The
given reaction is:
2H2O2(l)
→ 2H2O (l) + O2 (g)
Using the enthalpy of formation
values given in the question, we can calculate the enthalpy change for this
reaction as follows:
Enthalpy of reactants = 2 × (-188
kJ/mol) = -376 kJ/mol
Enthalpy of products = 2 × (286
kJ/mol) + 0 kJ/mol = 572 kJ/mol
Enthalpy change (ΔH) = Enthalpy
of products - Enthalpy of reactants
Or ΔH = (572 kJ/mol) - (-376
kJ/mol) = 948 kJ/mol
Therefore, the enthalpy change of
the given reaction is 948 kJ/mol. This means that the reaction is exothermic
and releases energy in the form of heat.
13. Ionic Product of
water at 250C is 1×10-14 and water are regarded
as very weak electrolyte.
i.
Define ionic product of water.
Ans: The ionic product of
water, denoted by Kw, is a measure of the concentration of hydrogen ions (H+)
and hydroxide ions (OH-) in pure water at a particular temperature.
ii.
Deduce the relation Kw=[H+]
[OH].
Ans: The dissociation
equilibrium of water is
H2O ⇆
[H+] [OH-]
Applying the law of mass action
under equilibrium condition
$K = {\rm{ }}\frac{{\left[ {{H^ +
}} \right]{\rm{ }}\left[ {O{H^ - }} \right]}}{{{H_2}O}}$
K.[ H2O] = [H+]
[OH-]
i.e. Kw=[H+]
[OH-]
iii.
Calculate the [OH-] concentration
of 0.01M HCl at 250C
Ans: HCl is a strong acid
that completely dissociates in water, so the concentration of H+ ions in the
solution will be equal to the concentration of the acid, which is 0. 01 M.
Thus, [H]+=[HCl]=0.01 M.
But [H]+[OH]−=kw=10−14
Hence,
[OH]−=kw/[H]+
=10−14/0.01
=10−12 M.
iv.
What is the effect of temperature on ionic
product of water?
Ans: With the increase in
temperature, there is increase in concentrations of these ions and hence the
value of the ionic product also increases.
14. Ethyl alcohol is a
common alcohol and is used to manufacture alcoholic beverage. It can be
prepared from sugar containing materials like molasses by fermentation
processes.
i.
Define Fermentation.
Ans: Fermentation is a
metabolic process that converts sugar or other organic compounds into simpler
compounds, such as alcohol or lactic acid, in the absence of oxygen.
ii.
What is meant by molasses?
Ans: Molasses is a thick,
dark syrup that is produced during the process of refining sugar from sugarcane
or sugar beets. It is a byproduct of the sugar-making process, and is composed
mainly of sugars, including sucrose, glucose, and fructose.
iii.
Mention the function of yeast in the
formation of ethyl alcohol.
Ans: During fermentation,
yeast breaks down the complex sugar molecules into simpler molecules such as
glucose and fructose. Yeast then converts these simpler molecules into ethanol
and carbon dioxide through a series of enzymatic reactions.
iv.
Write chemical reaction for the conversion of
cane-sugar into ethyl alcohol.
Ans: The chemical reaction
for the conversion of cane sugar (sucrose) into ethyl alcohol (ethanol) is:
C12H22O11
(sucrose) + H2O → C6H12O6 (glucose)
+ C6H12O6 (Fructose)
C6H12O6
(glucose) → 2C2H5OH (ethanol) + 2CO2 (carbon
dioxide)
v.
Give a difference between absolute alcohol
and denatured alcohol.
Ans: The key difference
between absolute alcohol and denatured alcohol is their purity and chemical
composition.
|
Absolute Alcohol |
Denatured Alcohol |
|
At least 99.9% pure ethanol, with the remaining 0.1% consisting of
water. |
Ethanol adulterated with other chemicals. |
|
High purity |
Lower purity than absolute alcohol |
|
Laboratory and industrial applications |
Solvent, fuel, and other industrial applications |
|
Low toxicity. |
Higher toxicity than absolute alcohol due to the presence of other
chemicals |
|
Typically, available through specialized suppliers |
Widely available in hardware and home improvement stores |
15. A carbonyl compound
(M) contains three carbon atoms, and it undergoes iodoform test.
i.
Identify the compounds (M)
Ans: Compound M is
Propanone
ii.
Write down the chemical reaction for the
preparation of (M)
Ans:
${\rm{C}}{{\rm{H}}_{\rm{3}}}\mathop {{\rm{ - CH}}}\limits^{\mathop |\limits^{OH} } {\rm{ - C}}{{\rm{H}}_{\rm{3}}}{\rm{ }} + {\rm{ [O] }}\mathop \to \limits^{KMn{O_4}} {\rm{ C}}{{\rm{H}}_{\rm{3}}}\mathop {{\rm{ - C}}}\limits^{\mathop {||}\limits^O } {\rm{ - C}}{{\rm{H}}_{\rm{3}}}\,$2 propanol Propanone
iii.
How is (M) converted into propane?
Ans: ${\rm{C}}{{\rm{H}}_{\rm{3}}}\mathop {{\rm{ - C}}}\limits^{\mathop {||}\limits^O } {\rm{ - C}}{{\rm{H}}_{\rm{3}}}\,{\rm{ + }}\,{\rm{[H] }}\mathop {\mathop \to \limits^{N{H_2}N{H_2}} }\limits_{KOH} {\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}} - {\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{ }}$
Propanone Propane
iv.
Predict the final product obtained when (M)
is treated with CH3MgI in presence of dry ether and followed by
hydrolysis?
Ans: $\begin{array}{l}{\rm{C}}{{\rm{H}}_{\rm{3}}}\mathop {{\rm{ - C}}}\limits^{\mathop {||}\limits^O } {\rm{ - C}}{{\rm{H}}_{\rm{3}}}\,\; + \,C{H_3}MgI\mathop \to \limits^{Dryether} {\rm{C}}{{\rm{H}}_{\rm{3}}} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{OMgI} } - {\rm{C}}{{\rm{H}}_{\rm{3}}}\\(\Pr opanone){\rm{ }}\end{array}$
${\rm{C}}{{\rm{H}}_{\rm{3}}} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{OMgI} } - {\rm{C}}{{\rm{H}}_{\rm{3}}}\mathop \to \limits^{{H_2}O} {\rm{C}}{{\rm{H}}_{\rm{3}}} - \mathop C\limits_{\mathop |\limits_{C{H_3}} }^{\mathop |\limits^{OH} } - {\rm{C}}{{\rm{H}}_{\rm{3}}} + Mg(OH)I{\rm{ }}$
2 methyl propan-2-ol
v.
Give a laboratory test reaction of carbonyl
compound.
Ans: ${\rm{C}}{{\rm{H}}_{\rm{3}}}\mathop {{\rm{ - C}}}\limits^{\mathop {||}\limits^O } {\rm{ - C}}{{\rm{H}}_{\rm{3}}}{\rm{ + NaOH + }}{{\rm{I}}_{\rm{2}}}{\rm{ }} \to {\rm{ CH}}{{\rm{I}}_{\rm{3}}} \downarrow {\rm{ + C}}{{\rm{H}}_{\rm{3}}}\mathop {{\rm{ - C}}}\limits^{\mathop {||}\limits^O } - ONa$
Propanone Iodoform
OR
Convert
ethoxy ethane from a haloalkane C2H2Br by using
Williamson’s reaction.
C2H5
+ CH3-CH2-ONa → C2H5-O-C2H5
+NaBr
i.
What product is obtained when ethoxyethane is
heated with excess HI.
Ans: When ethoxyethane is
heated with excess HI, ethanol and iodomethane are obtained as products. The
reaction can be represented as follows:
C2H5OC2H5
+ 2HI → C2H5OH + CH3I + H2O
ii.
Why are world sample of ether not distilled
to dryness in air.
Ans: Ether is highly
volatile and flammable. When it is distilled to dryness in air, it can form
explosive peroxides, which can be hazardous. Therefore, it is recommended to
store ethers with an antioxidant to prevent the formation of peroxides.
iii.
Convert phenol into anisole.
Ans: Anisole can be
prepared by the reaction of phenol with methyl iodide in the presence of a
strong base such as sodium hydride. The reaction can be represented as follows:
C6H5OH (phenol)
+ CH3I (iodo-ethane) → C6H5OCH3 (anisole)
+ HI
16. For the following reaction sequence.
i. Write down reagent and conditions for
reaction (1), reaction (2), reaction (3) and reaction (4).
Ans: Various condition in
above reaction is:
1: Heating at 2700 C
2: Carbon Dioxide (CO2)
3: Carbon Monoxide (CO)
4: Heating with P2O5
ii.Identify the compound (z) giving IUPAC name.
Ans: Z is Propanol: CH3-CH2-CH2-OH
17. How would you apply
Hoffmans’ method for the separation of 10 20 and 30
amine from their mixture?
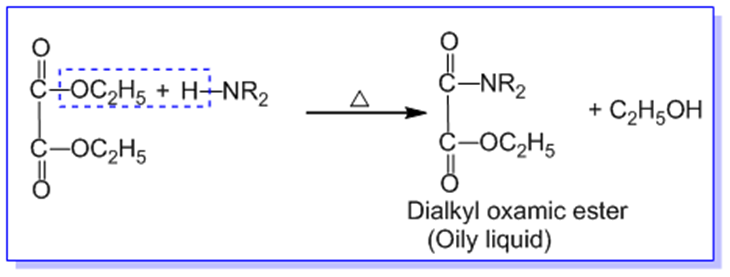
Ans: In
Hoffmann’s method, the given mixture of primary, secondary, and tertiary amines
is heated with diethyl oxalate when,
A.
10 amine forms dialkyl oxamide
which is crystalline solid.
B.
20 amine forms dialkyl oxamic
ester which is an oily liquid.
C.
30 amines do not react as it
does not contain replaceable hydrogen atom on nitrogen.
The reaction
mixture containing dialkyl oxamide, dialkyl oxamic ester, tertiary amine and
ethyl alcohol is first filtered and solid product of dialkyl oxamide is
separated. Dialkyl oxamide is heated with aq.KOH to recover primary amine.
The remaining
mixture of dialkyl oxamic ester, tertiary amine and ethyl alcohol is subjected
to fractional distillation. Tertiary amine is distilled out first. The residual
dialkyl oxamic ester is heated with aq. KOH to recover secondary amine and
alcohol in different fractions.
In this way,
the given mixture of 10,20 and 30 amines
are separated by Hoffmann’s method.
18. An important
compound non-typical transition metal zinc which is used as a lotion and is
also called white vitriol.
i. Write down the method for the preparation of
white vitriol.
Ans: The method for the
preparation of white vitriol involves the reaction of zinc oxide or zinc metal
with dilute sulfuric acid. The reaction can be represented as follows:
Zn + dil. H2SO4
→ ZnSO4 + H2
\[ZnS{O_4}\mathop {\mathop \to \limits^{Crystallization}
}\limits_{Po{\mathop{\rm int}} } {\rm{ }}ZnS{O_4}.7{\rm{ }}{H_2}O\]
ii. What happens when white vitriol is heated to
8000C?
Ans: White vitriol changes
to zinc oxide and releases Sulfur dioxide gas when heated.
$ZnS{O_4}\mathop \to \limits_\Delta ^{{{800}^0}C} {\rm{
}}ZnO{\rm{ }} + {\rm{ }}S{O_2}$
iii.
Define double salt giving an example of it.
Ans: A double salt is a
type of salt that contains two or more different cations or anions, each of
which is normally found in a separate salt. Double salts have a unique chemical
formula and crystalline structure.
An example of a double salt is
Mohr's salt, which has the chemical formula FeSO4(NH4)2SO4·6H2O.
iv. How is Lithopone obtained from white vitriol.
Ans: Lithopone is obtained
from white vitriol by the reaction of zinc sulfate with barium sulfide. The
reaction can be represented as follows:
ZnSO4 + BaS → ZnS-BaSO4
v. Why is zinc considered as non-typical
transition metal.
Ans: Zinc is considered a
non-typical transition metal because it has a full d-subshell in its most
stable oxidation state (Zn2+), unlike other transition metals that have
partially filled d-subshells. This gives zinc properties that are more like the
alkaline earth metals than to the transition metals.
19. Steel manufactured
by open hearth process.
i. What is open Hearth Process?
Ans: The Open-Hearth
Process is a method of producing steel from pig iron and scrap metal by melting
them together with a flux in a rectangular furnace lined with firebrick, and
then lowering the carbon content by oxidizing the impurities with air blown
through the molten metal. It was a popular method of steel production in the
early 20th century but has been largely replaced by more efficient methods.
ii. Write down the chemical reaction occurring in
Open-Hearth furnace.
Ans: Reaction involved in
open Hearth furnace are:
Fe2O3 + S →
Fe + SO2
Fe2O3 + P →
Fe + P2O5
Fe2O3 + Si →
Fe + SiO2
CaO + SiO2→CaSiO3
CaO + P2O5→Ca3(PO4)2
iii. Why is spiegeleisen added in the Open-hearth
furnace?
Ans: Spiegeleisen is an
iron alloy containing manganese and carbon, and it is added to the open-hearth
furnace during steel production because it helps to reduce the amount of carbon
in the steel and increases its manganese content.
iv. Write down the composition of stainless
steel.
Ans: Stainless steel is a
type of steel alloy that contains different metals like:
- Chromium (Cr): 17-19%
- Nickel (Ni): 7-9%
- Carbon (C): 0.08%
- Iron (Fe): 72%
Group C
20. a) Write an example
of each of the following reactions.
i. Hydroboration oxidation
Ans:
${\rm{C}}{{\rm{H}}_{\rm{3}}} - CH = {\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{ + (B}}{{\rm{H}}_{\rm{3}}}{{\rm{)}}_{\rm{2}}} \to {\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{ - B}}{{\rm{H}}_{\rm{2}}}$1 propene
and
${\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{ - B}}{{\rm{H}}_{\rm{2}}}\mathop \to \limits^{{H_2}{O_2}/O{H^ - }} {\rm{C}}{{\rm{H}}_{\rm{3}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}} - {\rm{C}}{{\rm{H}}_{\rm{2}}} - OH{\rm{ + B(OH}}{{\rm{)}}_{\rm{3}}}{\rm{ }}$
propan-1-ol
ii. Decarbonylation:
Ans:
2CH3COONa (Sodium
Acetate) + CaO (Calcium oxide) → CH4 (Methane)+ Na2CO3
+ CaCO3
iii. Sandmeyer’s reaction
Ans:
iv. Iodoform reaction
Ans: CH3CHO
(Ethanol) + I2 + NaOH → CHI3 (Iodoform) + HCOONa
v. Elimination reaction
Ans:
CH3-CH2-Br (Brome
Ethane) + alc.KOH → CH2=CH2 (Ethene) + KBr
vi. Cannizzaro’s reaction
Ans:
vii. Reimer-Tiemann reaction
Ans:
viii. Friedel Craft alkylation
Ans:
OR
An Unsaturated hydrocarbon (C3H6)
undergoes Markovnikov’s rule to give (A). Compound (A) is hydrolyzed with aq.
Alkali to yield (B). When (B) is treated with PBr3, Compound (C) is
produced. (C) reacts with alc. AgCN to give another compound (D). The compound
(D) if reduced with LiAlH4, Produce (E).
i. Define Markovnikov’s rule.
Ans: Markovnikov's rule is
a principle that governs the addition of hydrogen halides to asymmetric
alkenes, stating that the hydrogen atom will add to the carbon atom with more
hydrogen atoms and the halogen atom will add to the carbon atom with fewer hydrogen
atoms.
ii.
Identify (A), (B), (C), (D) and (E) with
chemical reaction.
Ans: The complete reaction is
iii.
How does E react with nitrous acid?
Ans: The complete reaction is
${\rm{C}}{{\rm{H}}_{\rm{3}}} - \mathop {CH}\limits^{\mathop |\limits^{NH - C{H_3}} } - C{H_3}{\rm{ (E) + HN}}{{\rm{O}}_{\rm{2}}} \to C{H_3} - \mathop {CH}\limits^{\mathop |\limits^{\mathop {O = N - N}\limits^{\mathop |\limits^{C{H_3}} } } } - C{H_3}$
iv.
How would you convert (B) into C3H8?
Ans: The complete reaction
is:
21. a) For a
hypothetical chemical reaction m P + n Q → z; the rate law is, rate = K[P]m[Q]n.
Where K is rate constant of the reaction (m + n) are overall order.
i.
Define rate law.
Ans: Rate law, also known
as the rate equation, is a mathematical expression that describes the rate of a
chemical reaction in terms of the concentration of reactants.
ii.
Why is rate law experimental parameter?
Ans: The rate law is an
experimental parameter because it cannot be predicted solely based on the
stoichiometry or balanced equation of a chemical reaction. Instead, the rate
law must be determined experimentally by measuring the rate of the reaction
under different conditions and determining how changes in the concentrations of
reactants or other factors affect the rate of the reaction.
iii.
What is meant by rate constant?
Ans: The rate constant (k)
is a proportionality constant that appears in the rate law equation and relates
the rate of a chemical reaction to the concentrations of the reactants and any
catalysts present in the reaction.
iv.
Mention a difference between order and
molecularity of reaction.
Ans: The differences between order and molecularity
of a reaction:
|
Order |
Molecularity |
|
The sum of the exponents of the concentration terms in the rate law
equation |
The number of molecules that collide to form the reaction's activated
complex |
|
Dimensionless |
Usually expressed as integers |
|
Experiment |
Reaction mechanism |
|
The effect of concentration on the reaction rate |
The probability of a reaction occurring in a single collision |
|
Elementary reactions and overall reactions |
Only elementary reactions |
|
Can have fractional or complex values |
Only takes on integer values |
b) For the above reaction the
order of a reaction with respect to P&Q are first order and zero order
respectively. Experimental data obtained from the reaction are as below.
|
Expt. |
[P]M |
[Q]M |
Initial rate (M.sec-1) |
|
I |
0.1 |
0.1 |
2×10-2 |
|
II |
(A) |
0.2 |
4×10-2 |
|
III |
0.4 |
0.4 |
(C) |
|
IV |
(B) |
0.2 |
2×10-2 |
i)
Identify the value of A, B, and C.
ii)
Calculate the rate constant [k].
Ans:
We can use the rate law equation to set up a system of equations with the
experimental data given in the question. The rate law equation for the reaction
is:
Rate (R) = k[P]1[Q]
0 = k[P]
where k is the rate constant and
the exponents of [P] and [Q] represent their respective orders.
Using the given experimental
data, we can set up the following system of equations:
·
Experiment I: Rate(R) = k [0.1] = 2×10-2
M/s
·
Experiment II: Rate(R) = k[A] = 4×10-2
M/s
·
Experiment III: Rate(R) = k [0.4] = C M/s
·
Experiment IV: Rate(R) = k [B] = 2×10-2
M/s
Using Experiment-I, we can
obtain a value for k:
\[k = \frac{{Rate}}{P} = \frac{{2
\times {{10}^{ - 2}}}}{{0.1}} = 0.2{M^{ - 1}}{s^{ - 1}}\]
Solving each equation for the
corresponding unknown variable, we can obtain the values of A, B, and C:
- Solving for A in Experiment II:
\[\begin{array}{l}\frac{R}{k} =
A\\or,\;{\rm{A = }}\frac{{4 \times {{10}^{ - 2}}}}{{0.2}} = 0.2\end{array}\]
- Solving for B in Experiment IV:
\[\begin{array}{l}\frac{R}{k} =
B\\or,{\rm{ B = }}\frac{{2 \times {{10}^{ - 2}}}}{{0.2}} =
0.1\end{array}\]
- Solving for C in Experiment III:
\[\begin{array}{l}k \times 0.4 =
C\\C = {\rm{ }}0.2 \times 0.4 = 0.08\end{array}\]
Thus, the final values are
- A = 0.4 M
- B = 0.1 M
- C = 0.08M/s
OR
a)
Crystal of oxalic acid is generally Used
to prepare primary standard solution.
I.
Define primary standard solution.
Ans: Primary standard
solutions are used to determine the concentration of an unknown substance by
titration, where a known volume of the primary standard solution is reacted
with a measured volume of the unknown substance until the reaction is complete.
II.
Which chemical indicator is used to
initiation of KMNO4 solution versus oxalic acid solution.
Ans: No chemical indicator
is used for the titration of potassium permanganate (KMNO4) versus oxalic
acid because the reactants themselves act as their own indicators. The endpoint
is detected visually by observing the disappearance of the purple color of the
potassium permanganate solution.
III.
Why is oxalic acid solution warmed adding
dilute H2SO4 before tight rating with KMNO4?
Ans: Heating the oxalic
acid solution to around 60°C increases the kinetic energy of the molecules,
leading to more frequent and energetic collisions that result in a faster
reaction between the oxalic acid and potassium permanganate. This increase in
temperature helps to accelerate the reaction rate and ensures a more efficient
and accurate titration.
IV.
Mention major application of titration in
quality control laboratory.
Ans: Some major
applications of titration in quality control laboratories:
- Titration is used to determine the acidity and
alkalinity of various products, such as food and beverages, cosmetics,
pharmaceuticals, and industrial chemicals.
- Titration is commonly used in the analysis of
pharmaceuticals to determine the concentration of active ingredients,
impurities, and other substances present in the product.
- Titration can be used to determine the concentration
of various substances in water, such as dissolved oxygen, chlorine, and
hardness.
- Titration can be used to determine the concentration
of various substances in food, such as salt, sugar, and acidity.
- Titration can be used to determine the concentration
of various substances in industrial chemicals, such as acids, bases, and
solvents.
b)
An aqueous solution of dibasic containing
17.7 gm of acid per liter of the solution, has density 1.0077gm/litre (molar
mass of the acid = 118gm/mol) Calculate i) molarity ii) molality
Ans:
Given:
Amount of acid (w) = 17.7 gm
Volume of solution (V) = 1 liter
Density (d) = 1.0077 gm/mL
Molar mass of acid (M) = 118
gm/mol
To calculate: i) Molarity ii)
Molality
Solution:
i) Molarity (M):
Molarity = Number of moles of
solute / Volume of solution in liters
Now,
Number of moles of solute
= weight of
solute / molar mass of solute Number of moles of solute
= 17.7 gm / 118
gm/mol
Number of moles of solute = 0.15
mol
Substituting the values in the
molarity equation, we get:
Molarity = 0.15 mol / 1 liter
Molarity
= 0.15 M
Therefore, the molarity of the
solution is 0.15 M.
ii) Molality (m):
Molality = Number of moles of
solute / Mass of solvent in kg
Now,
Mass of solution = Volume of
solution x Density of solution Mass of solution
= 1 L x 1.0077
gm/mL Mass of solution
= 1.0077 kg
Mass of the solute is 17.7 gm
We can calculate the mass of the
solvent as:
Mass of solvent = Total mass of
solution - Mass of solute Mass of solvent
= 1.0077 kg -
0.0177 kg Mass of solvent
= 0.99 kg
Now, we can calculate the
molality of the solution as:
Molality = 0.15 mol / 0.99 kg
= 0.152 m
Therefore, the molarity of the
solution is 0.15 M and the molality of the solution is 0.152 m.
22. A)
i.
What is Portland Cement?
Ans: Portland cement is a
hydraulic cement used in construction to bind materials together. It is made by
grinding clinker with a small amount of gypsum and is known for its strength,
durability, and ability to bond with other materials.
ii.
Name the major component present in Portland
cement.
Ans: The major components
present in Portland cement are:
Tricalcium silicate (3CaO · SiO2),
Dicalcium silicate (2CaO · SiO2),
Tricalcium aluminate (3CaO · Al2O3),
and
Tetra-calcium aluminoferrite
(4CaO · Al2O3Fe2O3)
iii.
Why is gypsum used in clinker during cement
production process?
Ans: Gypsum is added to
the clinker during cement production to control the setting time of the cement.
It reacts with tricalcium aluminate in the clinker to form a slow-setting
compound, which regulates the setting time and allows for shaping and
finishing. Gypsum also improves the workability of the cement and reduces water
needed in the mix.
iv.
Give any two instruments used for the quality
control cement.
Ans: Two instruments
commonly used for quality control in cement production are:
- X-ray fluorescence (XRF) spectrometer - This
instrument is used to analyze the chemical composition of raw materials,
clinker, and finished cement.
- Compressive strength tester - This instrument is used
to measure the compressive strength of cement.
B)
i.
Differentiate between homo-polymer and
co-polymer giving an example of each.
Ans: The difference
between homo-polymer and co-polymer is:
|
Homopolymer |
Copolymer |
|
Made up of a single type of monomer |
Made up of two or more different types of monomers |
|
Polymer chain consists of repeating units of the same monomer |
Polymer chain consists of repeating units of two or more different
monomers |
|
Generally, has more uniform properties and greater crystallinity |
Can exhibit a range of properties depending on the monomers used and
their proportions |
|
Example: Polyethylene made from ethylene monomers |
Example: ABS made from acrylonitrile, butadiene, and styrene monomers;
PPR made from propylene and ethylene monomers |
ii.
Name the monomers of the following
polymer and write their molecular formula.
a) Polystyrene
Ans: The monomer of
polystyrene is styrene. Its molecular formula is C8H8.
b) Bakelite:
Ans: The monomers of Bakelite are phenol and formaldehyde. Their molecular formulas are C6H5OH and CH2O, respectively.