ATOMIC STRUCTURE
Rutherford's
α-ray scattering experiment and nuclear model
In order to reveal the
arrangement of sub atomic particles in an atom, Rutherford and his associate
co-worker Geiger and Marsden performed a series of experiment with rays scattering
phenomenon on thin gold foil in 1911 which is, therefore, known as Rutherford's
α-ray scattering experiment.
In this experiment, a piece of a
radioactive substance like radium, uranium etc. was placed in a lead block
cavity and a rays produced from radioactive substance were allowed to pass
through a slate partition and bombarded on a thin gold foil of about 0.004mm
thickness around which a circular moveable ZnS coated screen was adjusted. When
the a-rays were scattered on the gold foil, they produced scintillation
(flashes of light) on the different portion of screen By examining and
observing the different portion of screen, it was became possible to determine
the various proportion of x-rays deflected from the gold foil with different
angle.
Observations:
From the above experiment,
following observations were made:
1. All most all (about 99%) of a-
rays passed through the gold foil without any appreciable deflection.
2. Few-rays were deviated with
smaller angle than 90°.
3. Very few (1 in 10,000 - 1,
00,000) of the z-rays deviated with extreme larger angle and even renounced
back to the original path.
Inference/Conclusion
of experiment:
On the basis of above
observations, Rutherford came to the following conclusions:
1. Since almost all (99%) of the
rays passed through gold foil without any appreciable deflection, it means an
atom is almost empty (hollow).
2. Since few α-rays were deviated
with small angle than 90", it means they approached near to the positively
charged mass concentrated in the nucleus.
3. Since only few (1 in
10,000-1,00,000) a particle were deviated with larger angle and even renounced
back to the origin path, it means these particles directly encountered with
small but heavy positively charged mass that occupies small space of the nucleus.
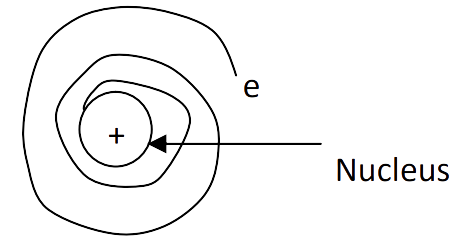
From the conclusion of a rays scattering experiment, Rutherford
proposed the nucleus model of atom as:
1. Most of the space in the atom
is empty.
2. Atom consists of positively
charged nucleus at which entire mass is concentrated.
3. Atom is electrically neutral
due to equal number of protons and electrons.
4. Nucleus is surrounded by
negatively charged electrons which revolve around the nucleus very high speed
like solar system.
Hence he proposed nuclear model
of an atom as given in figure
Limitation
of Rutherford's atomic model
1. Stability of an atom:
It could not explain the
stability of atom: If an electron revolves around the positively charged
nucleus as explained by Rutherford, it losses energy in the form of radiation
and its speed decreases due to which its path becomes spirally smaller and
smaller. Ultimately the electron falls in to the nucleus. However, it does not
happen.
Fig: Spiral path of
moving electron
2). It does not explain about emission of atomic spectrum.
Bohr's
Atomic Model
In 1913, a German scientist Neils
Bohr proposed a new atomic model with new concept in order to overcome the
drawbacks of Rutherford's atomic model by applying Planck's quantum theory of
radiation which is called Bohr's atomic theory. He retained the main spirit of
Rutherford's nuclear model.
The
major postulates of Bohr's atomic model are:
1. Stationary State:
Electrons revolve around the nucleus in fixed circular paths at certain distance from the nucleus which are called shells, stationary states, energy levels, orbits which are represented by K, L, M, N.... etc.
2. Numbers of electrons in a shell (2n2 formula):
The numbers of electrons that can
be hold in a shell is determined by 2n2 formula which is called Bohr-Bury rule. Here, n is number of
shell.
For example,
Number of electrons in first
shell- 2n2=2(1)2 Number of electrons in second shell-2(2)2-8
3. Quantization of angular momentum:
Only those orbits are permitted
for revolving electrons in which the angular momentum of the revolving electron
is equal to whole number multiple of nh/2π these orbits are called Bohr's
orbit.
mvr =
Where,
m: mass of electron
v: velocity of electron
r: radius of orbit
n=Number of shell
h: Planck’s constant(6.626 X 10-34JS)
4. Origin of spectral line (Atomic spectra):
When electron absorbs energy, it
jumps from lower energy level (orbit) to higher energy levels where it is
unstable and jumps back to the same lower energy level by emitting energy in
the form of light of different wave length that results production of spectral
lines. The quantity of absorbed or emitted energy is calculated as:
ΔE = E2 - E1
hv= E2 - E1
Where,
E1: energy of electron
in first shell
E2: energy of electron
in second shell.
ΔE: absorbed or emitted energy
H: Plank's constant
v: frequency of radiation
5. Balance of electrostatic force of attraction:
Electron revolves around the
nucleus in a fixed orbit at certain distance from nucleus is balanced by equal
but opposite force i.e. centripetal force and centrifugal force due to which
electron doesn't jump into the nucleus.
6. When an electron revolves in a
particular orbit, it neither radiates (looses) nor absorbs the energy at
ordinary condition.
Outcomes
(benefits) Bohr's atomic model
1. It answers successfully about
the stability of atom since electron neither losses nor absorbs energy in a
particular orbit is balanced by equal and opposite force centripetal
and centrifugal force.
2. It explains the origin of
atomic spectra in uni-electron system like H, Li++ etc.
3. It helps to calculate the
energy of the electron in a particular orbit of mono electronic system like
hydrogen, lithium. It can be calculated by
Where,
ε0: permittivity of
vacuum =8.85 x 1012 Fm‑1
Z: Atomic number
H: Planck’s constant=6.626 X 10-34
M: mass of electron (9.1x 10g)
e: charge of electron (-1.6 x10-19C)
n=principal quantum number or number of shell
5).It helps to calculate the
radius of the orbit by the relation
Where
r: radius of orbit
ε0: permittivity of
vacuum
Limitation
of Bohr's atomic model:
1. It is significant for the
origin of atomic spectra for uni electron system only like H, He",
Li" etc but failed for multi electrons system.
2. It does not explain about the
splitting of spectral lines in to fine lines in the high resolution
power.
3. It doesn't explain about the
splitting of spectral line into number of fine lines in the magnetic field (Zeeman
Effect) and in the electric field (Stark effect).
4. It fails to explain wave
nature of electron.
5. It is against of Heisenberg's
uncertainty principle and de-Broglie's equation.
6. It fails to account the three
dimensional arrangement of electrons.
7. It is unable to interpret
relationship of mvr= properly.
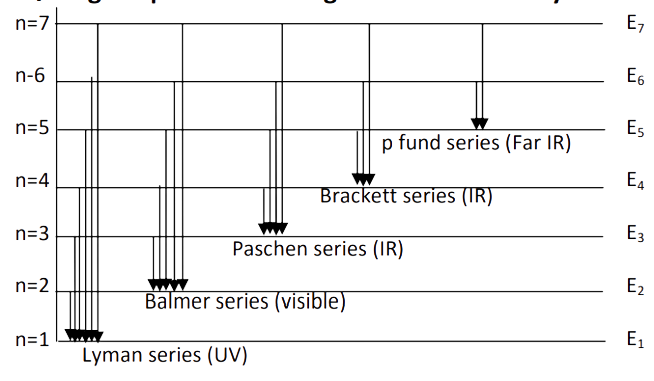
Hydrogen spectra in the light of Bohr's theory:
Fig: Hydrogen Spectra
Neils Bohr discovered spectra in
uni electron system like H by passing high voltage electricity under reduced
pressure. When high voltage electric discharge (10,000Volts) is applied to the
hydrogen gas under reduced pressure (0.001atm), hydrogen molecules dissociate
into hydrogen atoms at first, Electron of each hydrogen atom absorbs different
quantity of energy and jumps from its ground state to different higher energy
levels. These excited electrons are unstable at higher energy levels and return
back to the ground state either by direct single jump or more successive jumps
when the supply of energy cut off. As these excited electrons jumps back to the
ground state, they emit energy in the form of radiation having different wave
length which results the formation of series of spectral lines. Depending upon
the wave length of radiation, five different types of spectral series are recognized.
1. Lyman
series:
The spectral series observed when
the excited electron jumps from higher energy levels (n2=2, 3, 4, 5....etc.)
to the first energy level (n1=1) is called Lyman series. It
lies in ultraviolet region and wave length of radiation ranges from 920 A° to
1200 A°. Wave number of this series is determined by the relation.
Where,
λ: Wave length
v: wave number
R: Rydberg's constant =109677/cm
n1: Lower energy
level
n2: Higher energy
level
2.
Balmer Series:
The spectral series observed when
the excited electron jumps from higher energy levels (n2=3, 4, 5,
6...etc.) to the second energy level (n1=2) is called Balmer
series. It lies in visible region and wave length of radiation ranges from
4000 A° to 6500 A°. Wave number of this series is determined by the
relation.
3.
Paschen series:
The spectral series obtained when the excited electron jumps from higher energy levels (n2=4, 5, 6, 7....etc.) to the third energy level n1=3) is called Paschen series. It is observed in Infrared region and wave length of radiation ranges from 9500 A° to 18750 A°. Wave number of radiation of this series is determined by the relation.
4.
Brackett series:
The spectral series obtained when
the excited electron jumps from higher = 5, 6, 7...e energy levels (n2)
to the lower energy level (n1=4) is called Brackett series. It
lies in Infrared and wave length of radiation ranges from 19450 A° to 40500A°. Wave
length of radiation of this series is determined by the relation.
5. Pfund
Series:
The spectral series observed when
the excited electron jumps from higher energy levels (n2=6, 7....etc.)
to the lower energy level (n1=5) is called Pfund series. It
lies in Infra-red and wave length of radiation is above 40500A°. Wave length of
radiation of this series is determined by the relation.
Therefore types of series, range
of wavelength and their regions are summarized in the table:
|
Types of Series |
Wave length range |
Regions |
|
Lyman |
920Ao -1200Ao |
UV region |
|
Balmer |
4000Ao - 6500Ao |
Visible Region |
|
Paschen |
9500Ao -18750Ao |
Infra-Red Region |
|
Brackett |
19450Ao -40500Ao |
Infra-Red Region |
|
Pfund |
Above 40500Ao |
Far Infra-Red Region |
Quantum
Mechanics:
To overcome the uncertainty of
Bohr’s atomic model this theory was developed. This theory become successful to
explain the wave particle nature of the electron.
1. De
Broglie Wave Equation:
De-Broglie extended the idea of
dual nature of light to matter particle and suggested that all matter particle
in motion shows dual nature i.e. particle and wave nature. It means electron
shows the characteristics of wave as well as particle.
De Broglie Wave Equation: λ=
2.
Heisenberg Uncertainty Principle:
Statement: It states that it is impossible to
determine the position and momentum of microscopic particle in motion
simultaneously and accurately.
Mathematically: Δx. Δp >
Where,
Δx: Uncertainty in Position
Δp: Uncertainty in Momentum
3.
Schrödinger’s Wave Mechanics:
Statement: The wave particle duality of electron has laid foundation of wave mechanics model of an atom. He derived a complicated mathematical equation. Considering an atom as positively charged nucleus surrounded by stationary electron wave which extends around the nucleus in Bohr’s circular orbit. In this model electron is considered as three dimensional wave in electronic field of positively charged nucleus.
Difference
between orbit and orbital:
|
Orbit |
Orbital |
|
It is well defined circular path where electron revolves around
nucleus. |
It is the three dimensional space where the probability of finding
electron is maximum. |
|
The concept was developed from Bohr’s Atomic Model. |
The concept was developed from Heisenberg uncertainty principle. |
|
Orbit use two dimensional concept. |
Orbital use three dimensional concept. |
|
They are non-directional character. |
They use directional character. |
|
One orbit can accommodate maximum 2n2 number of electron. |
One orbital can accommodate only two electron. |
Quantum
Numbers:
Those number which gives complete information about the
motion and location of an electron in an atom is called quantum numbers.
There are altogether
four quantum numbers.
i.
Principle Quantum Number (n):
It represent Bohr’s orbit and
denotes major shell to which the electron belongs. The value of n is non-zero
positive integers and is equal to 1, 2, 3, 4… or K, L, M, N…. respectively.
The maximum number of electron that
a shell can hold is given by 2n2rule.
Example:
For K shell: maximum number of
electron = 2n2 = 2×12=2
ii.
Azimuthal Quantum Number(L):
It represent the number of subshell
by which a shell is composed of. The value of L is equal to 0, 1, 2, and 3 for
s, p, d and f orbitals respectively. Its value ranges from 0 to (n-1).
The maximum number of electron that a shell
can hold is given by 2n (2L +1) rule.
Example:
For s-subshell: maximum no. of
electron = 2n (2L +1) = 2×1 (2×0 +1) =2
Note:
S àSharpe
P à
Principle
D àDiffused
F à
Fundamental
iii. Magnetic
Quantum Numbers (M):
It helps to determine the
orientation of orbitals in space. The maximum number of electron that an
orbital can hold is 2.The value of M range from –L to L including 0.
Example:
For s-subshell: L=
0 and M= -0 to +0 i.e. 0. It means s subshell consist of single orbitals.
For p-subshell: L=1 and M = -1 to
1 i.e. -1, 0, 1. It means p-subshell consist of three orbital i.e. px,
py, pz
iv. Spin
Quantum Number (s):
While revolving around nucleus
electron circulates in its own axis either in clockwise or in anticlockwise
direction which gives information about the spin quantum number.
Example:
S=½ à
clockwise direction
S=-½à
anticlockwise direction
Electronic
Configuration (nLx):
1. Afbau
Principle:
It states that, “While filling
electrons in atomic orbitals, first filled up to the orbitals having lower
energy than only filled up to the orbitals having high energy.”
Fig.
Filling Sequence of electron in increasing of energy level
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,….
The orbital with
lower (n + L) value have lower energy and is filled first than the orbital with
higher energy.
Example: 2s orbital is filled earlier
than 2p orbital because 2s has lower (n +L) value than 2p orbital.
n+ L for 2s =
2+0=2
n + L for 2p =
2+1=3
If the orbital
have same (n + L) value than the orbital with lower n value is filled first.
Example: 3d orbital is filled earlier
than 4p orbital because 3d has lower value of n than 4p.
Electronic
Configuration of Element from 1 to 30:
|
Atomic Number |
Name of the Element |
Electronic Configuration |
|
1 |
Hydrogen (H) |
1s1 |
|
2 |
Helium (He) |
1s2 |
|
3 |
Lithium (Li) |
[He] 2s1 |
|
4 |
Beryllium (Be) |
[He] 2s2 |
|
5 |
Boron (B) |
[He] 2s2 2p1 |
|
6 |
Carbon (C) |
[He] 2s2 2p2 |
|
7 |
Nitrogen (N) |
[He] 2s2 2p3 |
|
8 |
Oxygen (O) |
[He] 2s2 2p4 |
|
9 |
Fluorine (F) |
[He] 2s2 2p5 |
|
10 |
Neon (Ne) |
[He] 2s2 2p6 |
|
11 |
Sodium (Na) |
[Ne] 3s1 |
|
12 |
Magnesium (Mg) |
[Ne] 3s2 |
|
13 |
Aluminum (Al) |
[Ne] 3s2 3p1 |
|
14 |
Silicon (Si) |
[Ne] 3s2 3p2 |
|
15 |
Phosphorus (P) |
[Ne] 3s2 3p3 |
|
16 |
Sulphur (S) |
[Ne] 3s2 3p4 |
|
17 |
Chlorine (Cl) |
[Ne] 3s2 3p5 |
|
18 |
Argon (Ar) |
[Ne] 3s2 3p6 |
|
19 |
Potassium (K) |
[Ar] 4s1 |
|
20 |
Calcium (Ca) |
[Ar] 4s2 |
|
21 |
Scandium (Sc) |
[Ar] 3d1 4s2 |
|
22 |
Titanium (Ti) |
[Ar] 3d2 4s2 |
|
23 |
Vanadium (V) |
[Ar] 3d3 4s2 |
|
24 |
Chromium (Cr) |
[Ar] 3d5 4s1 |
|
25 |
Manganese (Mn) |
[Ar] 3d5 4s2 |
|
26 |
Iron (Fe) |
[Ar] 3d6 4s2 |
|
27 |
Cobalt (Co) |
[Ar] 3d7 4s2 |
|
28 |
Nickel (Ni) |
[Ar] 3d8 4s2 |
|
29 |
Copper (Cu) |
[Ar] 3d10 4s1 |
|
30 |
Zinc (Zn) |
[Ar] 3d10 4s2 |
Electronic Configuration of IONS:
Ca++:1s22s22p63s23p6
Mg++:1s22s22p6
Fe+:1s22s22p63s23p64s13d6
Fe++:1s22s22p63s23p63d6
Fe+++:1s22s22p63s23p63d5
2.
Pauli’s Exclusion Principle:
It states that “No two electron of an atom has same set of
all four quantum number.”
Illustration: The quantum number of two
electron of helium are:
|
No. of electron |
n |
L |
m |
s |
|
1st electron |
1 |
0 |
0 |
+½ |
|
2nd electron |
1 |
0 |
0 |
-½ |
3.
Hund’s Rule of Maximum Multiplicity:
It states that,
“In the orbitals of same subshell
electron are filled singly first, before paring starts up.”
Electron are
arranged themselves in degenerated orbitals. So, as to retain parallel spin
quantum numbers. Electrons of same charge repels each other and tends to satay apart
from each other as far as possible. That means electron enter degenerated
orbitals, this minimizes inter electronic repulsion and the system will be more
stable due lowering of energy. Thus Hund’s rule accounts extra stability of
electronic configuration.
Importance of Hund’s Rule:
According to
Hund’s rule two electron in same orbital have different spin quantum number.
This verifies exclusion principle.
This rule is
applicable for knowing the electronic configuration in more expanded form.
Bohr
Bury Rule:
In 1921 Bohr's Bury proposed a
scheme for the distribution of electrons in different orbits in an atom of
the element is called Bohr's bury scheme.
The main points of this scheme are:
The maximum number of electrons that and hold is given by 2n² rule. Where n
is the number of shells. Therefore k shell poses 2 electrons, L shell poses 8
electrons, M shell poses 18 electrons, and N shell poses 32 electrons.
The maximum number of electrons in the outer orbit is 8 and next to the outer
most orbit that is penultimate orbit is 18.
Example:
|
Element |
Atomic No. |
K |
L |
M |
N |
O |
|
Kr |
36 |
2 |
8 |
18 |
8 |
|
|
Xe |
54 |
2 |
8 |
18 |
18 |
8 |
It is not necessary for an orbit to be completely filled before the next orbit
starts filling. In fact, new orbit starts electron filling when outermost orbit
get 8 electron.
Example:
|
Element |
Atomic No. |
K |
L |
M |
N |
|
Na |
11 |
2 |
8 |
1 |
|
|
K |
19 |
2 |
8 |
8 |
1 |